Search results
Search for "crown ethers" in Full Text gives 50 result(s) in Beilstein Journal of Organic Chemistry.
Switchable molecular tweezers: design and applications
Beilstein J. Org. Chem. 2024, 20, 504–539, doi:10.3762/bjoc.20.45

- sites was developed concomitantly to the nitrogen-based systems. In 1999, Boschi and co-workers reported the podand-based switchable tweezers 31 (Figure 17) [66]. This kind of spacer is flexible due to the ethylene glycol chain but can complex alkali cations like crown ethers. Upon complexation, the
Tying a knot between crown ethers and porphyrins
Beilstein J. Org. Chem. 2023, 19, 1630–1650, doi:10.3762/bjoc.19.120

- the possible future directions. Keywords: crown ethers; porphyrinoids; self-assembly; sensors; supramolecular chemistry; Introduction Many areas of modern supramolecular chemistry, organic, inorganic, materials and coordination chemistry, are based upon macrocyclic compounds of specifically-designed
- structures and tailored functions [1][2][3]. The design of novel macrocyclic compounds has laid the ground for phenomenal developments constituting supramolecular chemistry as a branch of chemical sciences. These fundamental developments included the discovery of crown ethers by Pedersen [4], followed by the
- materials science [10]. These two classes of molecules seem particularly remarkable among the endless family of macrocyclic compounds due to their unique features and easy accessibility. The popularity of porphyrins and crown ethers has been so extensive that the whole range of these macrocycles is even
Synthesis of α-(perfluoroalkylsulfonyl)propiophenones: a new set of reagents for the light-mediated perfluoroalkylation of aromatics
Beilstein J. Org. Chem. 2022, 18, 788–795, doi:10.3762/bjoc.18.79
- also employed crown ethers, 15-crown-5 and 18-crown-6, to test whether a “naked” sulfinate ion would help us achieve a better yield. Unfortunately, the addition of such ethers shut down all reactivity, most likely due to side reactions with the sulfinate salt. Moreover, it is worth mentioning that
Heteroleptic metallosupramolecular aggregates/complexation for supramolecular catalysis
Beilstein J. Org. Chem. 2022, 18, 597–630, doi:10.3762/bjoc.18.62

- the electrostatic interaction of crown ethers and alkali metals [4]. While, in the beginning, crown ethers were an excellent choice for metal ion complexation, they later received ample recognition as supramolecular catalysts [49]. The majority of host capsules, however, has been constructed using
Synthesis of a novel aminobenzene-containing hemicucurbituril and its fluorescence spectral properties with ions
Beilstein J. Org. Chem. 2021, 17, 2840–2847, doi:10.3762/bjoc.17.195

- Macrocycles with converging binding sites and functional groups hold a key position in supramolecular chemistry, which has been repeatedly confirmed by classic macrocyclic molecules, such as crown ethers, cyclodextrins, calixarenes, cucurbiturils and their homologues [1]. For the past decades, numerous
Insight into functionalized-macrocycles-guided supramolecular photocatalysis
Beilstein J. Org. Chem. 2021, 17, 139–155, doi:10.3762/bjoc.17.15

- with various macrocyclic hosts as well as on the role of macrocyclic-hosts-assisted hybrid materials in energy transfer. To keep the clarity of this review, the macrocycles are categorized into the most commonly used supramolecular hosts, including crown ethers, cyclodextrins, cucurbiturils
- supramolecular hosts (Figure 1); crown ethers (CEs), cyclodextrins (CDs), cucurbiturils (CBs), calixarenes (CAs), and pillararenes (PAs) for various photocatalytic reactions. However, biomolecules and other supramolecular-host-related photocatalytic reactions are not included in this minireview. Generally, a
- can easily gain information on host–guest interactions in supramolecular systems as well as the photocatalytic reaction efficiency. Review Macrocycle-promoted photocatalysis Photocatalysis based on crown ethers and crown ether derivatives CEs are a class of macrocycles that consist of a ring with a
Chiral anion recognition using calix[4]arene-based ureido receptors in a 1,3-alternate conformation
Beilstein J. Org. Chem. 2020, 16, 2999–3007, doi:10.3762/bjoc.16.249
- -containing onium salts, protonated or alkylated aza-crown ethers and azacryptands, amidinium and guanidinium cations, etc. [15][16][17][18]. Due to the low directionality of the Coulomb force, the successful application of purely ionic interactions in the design of selective anion receptors is rather limited
Thermodynamic and electrochemical study of tailor-made crown ethers for redox-switchable (pseudo)rotaxanes
Beilstein J. Org. Chem. 2020, 16, 2576–2588, doi:10.3762/bjoc.16.209

- Stuttgart, Pfaffenwaldring 55, 70569 Stuttgart, Germany Department of Chemistry, University of Jyvaskyla P. O. Box 35, 40014 Jyväskylä, Finland 10.3762/bjoc.16.209 Abstract Crown ethers are common building blocks in supramolecular chemistry and are frequently applied as cation sensors or as subunits in
- synthetic molecular machines. Developing switchable and specifically designed crown ethers enables the implementation of function into molecular assemblies. Seven tailor-made redox-active crown ethers incorporating tetrathiafulvalene (TTF) or naphthalene diimide (NDI) as redox-switchable building blocks are
- corresponding free macrocycle. The detailed understanding of the thermodynamic and electrochemical properties of the tailor-made crown ethers lays the foundation for the construction of new types of molecular redox switches with emergent properties. Keywords: crown ether; isothermal titration calorimetry
Design and synthesis of a bis-macrocyclic host and guests as building blocks for small molecular knots
Beilstein J. Org. Chem. 2020, 16, 2314–2321, doi:10.3762/bjoc.16.192
- . Keywords: alkynes; azides; host–guest; macrocycles; molecular knots; Introduction Macrocycles have played a central role in the development of molecular recognition, self-assembled molecular devices, and molecular topology [1][2][3][4][5][6]. For example, early work by Pedersen on crown ethers [1], Lehn
A dynamic combinatorial library for biomimetic recognition of dipeptides in water
Beilstein J. Org. Chem. 2020, 16, 1588–1595, doi:10.3762/bjoc.16.131

- , selective binders for peptides are extremely desirable but also highly challenging to design. Early developments in artificial peptide recognition go back to the 1970’s when it was discovered that crown ethers can bind to ammonium functions, such as protonated amines in peptides [1]. Further such binding
The use of isoxazoline and isoxazole scaffolding in the design of novel thiourea and amide liquid-crystalline compounds
Beilstein J. Org. Chem. 2020, 16, 175–184, doi:10.3762/bjoc.16.20
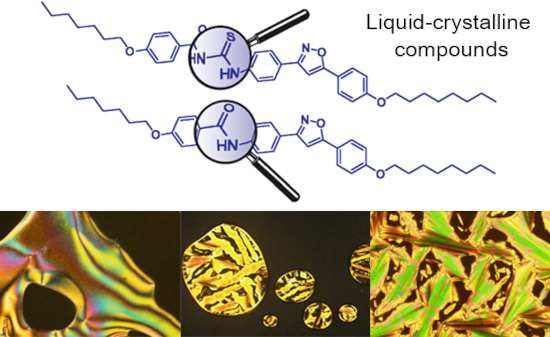
- and the amine 4 (Scheme 3, part B). Attempts to improve the reactivity of potassium cyanate by adding crown ethers did not result in the formation of ureas as expected. Thus, five new amides 19–22 and 24 were obtained in yield range of 42–71% (19 42%; 20 53%; 21 58%; 22 53%; 24 71%). Mesomorphic
The interaction between cucurbit[8]uril and baicalein and the effect on baicalein properties
Beilstein J. Org. Chem. 2020, 16, 71–77, doi:10.3762/bjoc.16.9
- compounds such as calixarenes, crown ethers and cyclodextrins, which can be used to form a stable inclusion complex with the drug, and improving the bioavailability of the drug [34][35]. Herein, we describe the results of the investigations of host–guest interactions between BALE and Q[8] in an aqueous
Photoreversible stretching of a BAPTA chelator marshalling Ca2+-binding in aqueous media
Beilstein J. Org. Chem. 2019, 15, 2801–2811, doi:10.3762/bjoc.15.273

- alternative approach to generate calcium release, where deforming an ion-binding site would render it less well-adapted to bind a guest. Photoisomerization of the azobenzene moiety has previously been successfully used to modulate the affinity of crown ethers [29], lariats [30], and foldamers [31] for various
1,2,3-Triazolium macrocycles in supramolecular chemistry
Beilstein J. Org. Chem. 2019, 15, 2142–2155, doi:10.3762/bjoc.15.211
- catenane 17b upon addition of base were less noticeable because of a weaker CT band in the case of a dibenzo-fused crown ether. Thus, the authors have proven that the movement of macrocyclic rings within catenanes can be controlled by switching the pH, and the bisnaphtho crown ethers can act either as
Multiple threading of a triple-calix[6]arene host
Beilstein J. Org. Chem. 2019, 15, 2092–2104, doi:10.3762/bjoc.15.207

- architectures. Historically, the most common components were dialkylammonium ions, as axles, and crown ethers, cyclodextrins, or cucurbiturils, as wheels [1]. With respect to the possible types of wheels, more recently, we have introduced the through-the-annulus threading of simple calix[6]arene hosts with
Tautomerism as primary signaling mechanism in metal sensing: the case of amide group
Beilstein J. Org. Chem. 2019, 15, 1898–1906, doi:10.3762/bjoc.15.185

- alkali metal determination is still a challenge. In the case of alkaline earth metal ions, the reagents with reasonable selectivity are still not commonly accepted since they compete with transitional metal ions [4]. The discovery of crown ethers [5] and 3D-based ligands [6] unquestionably helped the
Novel macrocycles – and old ones doing new tricks
Beilstein J. Org. Chem. 2019, 15, 1838–1839, doi:10.3762/bjoc.15.178

- 10.3762/bjoc.15.178 Keywords: macrocycles; supramolecular chemistry; Macrocycles [1] are the workhorses in supramolecular chemistry. Many basic supramolecular concepts have been developed through studying crown ethers, cryptands, podands and spherands in the 1970s and 1980s. For these contributions
Host–guest interactions between p-sulfonatocalix[4]arene and p-sulfonatothiacalix[4]arene and group IA, IIA and f-block metal cations: a DFT/SMD study
Beilstein J. Org. Chem. 2019, 15, 1321–1330, doi:10.3762/bjoc.15.131

- the well-studied cyclodextrins and crown ethers. Calixarenes are products of phenol–aldehyde condensation, as the aromatic components may derived from phenol, resorcinol, or pyrogallol; the aldehyde most often used for phenol is simple formaldehyde (methanal, HCHO), while larger aldehydes (e.g
Self-assembly behaviors of perylene- and naphthalene-crown macrocycle conjugates in aqueous medium
Beilstein J. Org. Chem. 2019, 15, 1203–1209, doi:10.3762/bjoc.15.117

- macrocyclic hosts, crown ethers, have been widely used as building blocks for supramolecular assemblies in the past two decades [37][38][39][40][41][42][43][44][45][46][47][48][49][50][51][52][53], mainly in the fabrication of crown ether-based threaded or interlocked assemblies in organic solvents [54
- water-soluble crown macrocycle 3 was synthesized according to reported protocols, by using potassium cations as template [86][87]. The primary amine group in 3 offers diverse reaction pathways to design crown ethers appended LMW gelators, dendrimers and polymers [88][89][90][91][92]. In order to
Precious metal-free molecular machines for solar thermal energy storage
Beilstein J. Org. Chem. 2019, 15, 1096–1106, doi:10.3762/bjoc.15.106

- , their structures predispose to an easy functionalization and low cost synthesis. Another advantage of these dyes is the very low molar absorptivity [16][17] of the higher energy cis isomer, which is a prerequisite for its photostability. The cis isomers of styryl dyes linked to crown ethers have been
- . This finding is a resemblance to the results from reference [24] where the 5-methoxy-substituted benzothiazole styryl-crown ethers demonstrated higher quantum yields of trans-to-cis photoisomerization. Insight from electronic structure calculations To rationalize the experimental findings, we performed
Design, synthesis and spectroscopic properties of crown ether-capped dibenzotetraaza[14]annulenes
Beilstein J. Org. Chem. 2019, 15, 617–622, doi:10.3762/bjoc.15.57

- much better understanding of complex biological systems [1]. Since the serendipitous discovery of crown ethers by Charles Pedersen in 1967 [2], there have been significant advances in the design and synthesis of sophisticated multidentate macrocycles with mixed-donor sites [3] and high selectivity
LCST phase behavior of benzo-21-crown-7 with different alkyl chains
Beilstein J. Org. Chem. 2019, 15, 437–444, doi:10.3762/bjoc.15.38

- .15.38 Abstract The introduction of hydrophobic units into crown ethers can dramatically decrease the critical transition temperature of LCST and realize macroscopic phase separation at low to moderate temperature and concentration. Minor modifications in the chemical structure of crown ethers (benzo-21
- -crown-7, B21C7s) can effectively control the thermo-responsive properties. Keywords: crown ethers; hydrophobic units; lower critical solution temperature; LCST; thermo-responsiveness; supramolecular chemistry; Introduction The introduction of stimuli-responsiveness into artificial materials is vital
- (>50 °C) are necessary to realize LCST phase separation, which significantly restricts an application in functional materials. In order to achieve LCST behavior of crown ethers at moderate concentrations and temperatures, two methods would be applied: combining multiple B21C7 units into a single
Tetrathiafulvalene – a redox-switchable building block to control motion in mechanically interlocked molecules
Beilstein J. Org. Chem. 2018, 14, 2163–2185, doi:10.3762/bjoc.14.190

- the [3]rotaxane 23 which bears two cofacially oriented TTF crown ethers on a divalent ammonium axle. The distance between the wheels is convenient for TTF-dimer interactions. In the non-switched neutral state of both TTFs, the wheels adopt a syn co-conformation caused by weak non-covalent interactions
A pyridinium/anilinium [2]catenane that operates as an acid–base driven optical switch
Beilstein J. Org. Chem. 2018, 14, 1908–1916, doi:10.3762/bjoc.14.165

- (Figure 2) was synthesized using a one-step, self-assembly procedure from two bis(pyridinium)ethane axles, two terphenyl spacers and two DB24C8 crown ethers, a [2]catenane with different recognition sites requires a stepwise approach involving the incorporation of each recognition site independently
Strong binding and fluorescence sensing of bisphosphonates by guanidinium-modified calix[5]arene
Beilstein J. Org. Chem. 2018, 14, 1840–1845, doi:10.3762/bjoc.14.157

- particularly suitable for the detection of analytes lacking chromophores. The key factor in IDA is the rational design of artificial receptors that are capable of binding analytes strongly and specifically. Calixarenes are the third generation of macrocyclic receptors after crown ethers and cyclodextrins. Due






























































































































































































































































































































